Adults who have already received at least one dose of a COVID-19 vaccine express broad openness to receiving a booster shot. A large majority of vaccinated adults (62% of the total public) say they would probably get a vaccine booster, if public health officials recommend an additional dose. A far smaller share of vaccinated adults (10% of the total public) say they would probably not get a vaccine booster. This adjustment more accurately reflects how these estimates of vaccine effectiveness are used in our model; that is, to reduce the infection fatality ratio and the infection hospitalization ratio . We used the average ratio of vaccine effectiveness for hospitalization compared to symptomatic disease combined from studies in the United Kingdom and Canada to modify the estimated effectiveness from the clinical trials for these vaccines.
We also used the vaccine effectiveness against severe disease for the Johnson & Johnson vaccine instead of the efficacy against all symptomatic disease using results from the clinical trial. The FDA also posted a statement titled "FDA Will Follow The Science On COVID-19 Vaccines For Young Children". CanSino Biologics' vaccine was co-developed with the Chinese military. This is based on a multi-country analysis first posted on Twitter by Faisal Sultan, Pakistan's health adviser, on February 8, 2021. The Phase III trial includes 30,000 participants and demonstrated 90.98% efficacy in preventing severe disease. CanSino has agreed to supply 35 million doses to Mexico and is in talks with Malaysia for 3.5 million shots.
Pakistan is running one of the largest trials and has contracted for 20 million shots. It is also working with the WHO for approval for the vaccine through the Covax program. It is also planning a trial with Russia to determine if swapping the second dose of Russia's Sputnik V vaccine with Can Sino would produce the same or better protection.
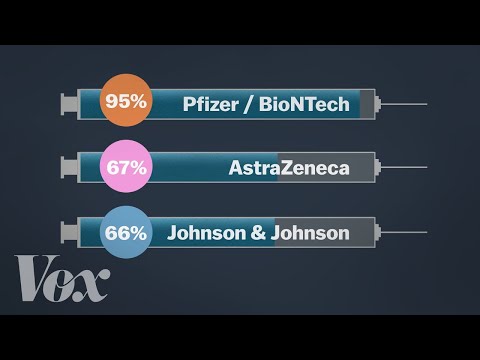
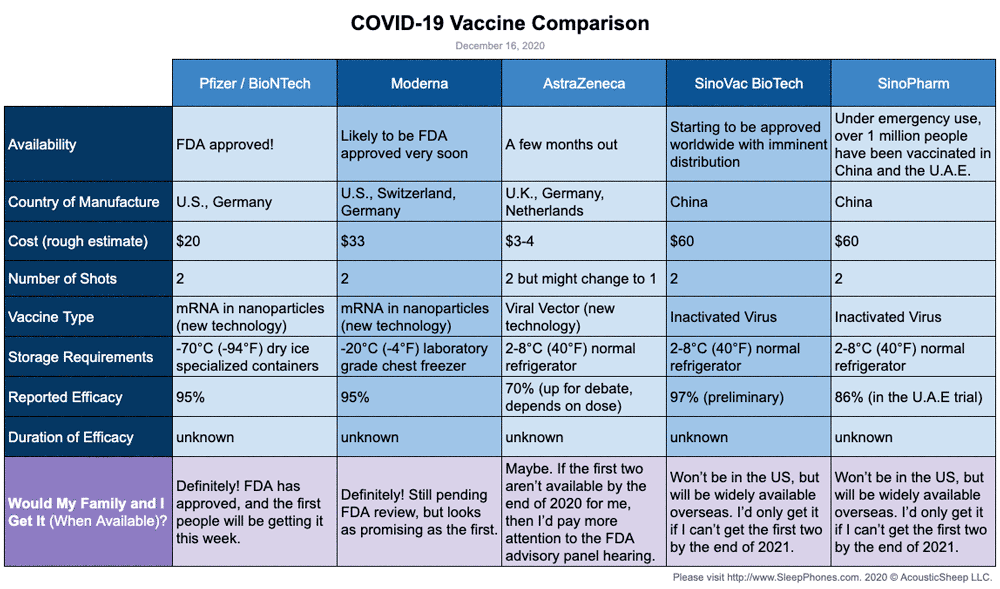
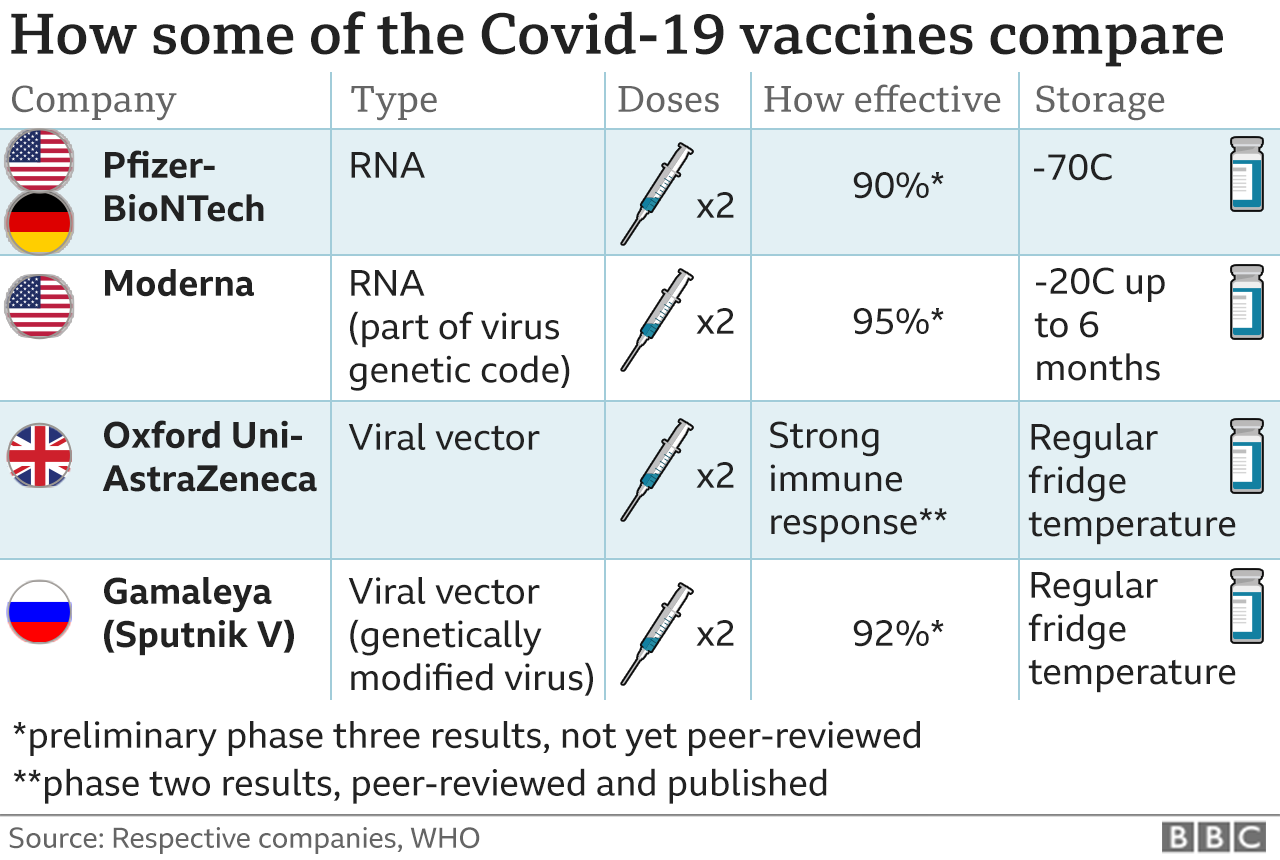
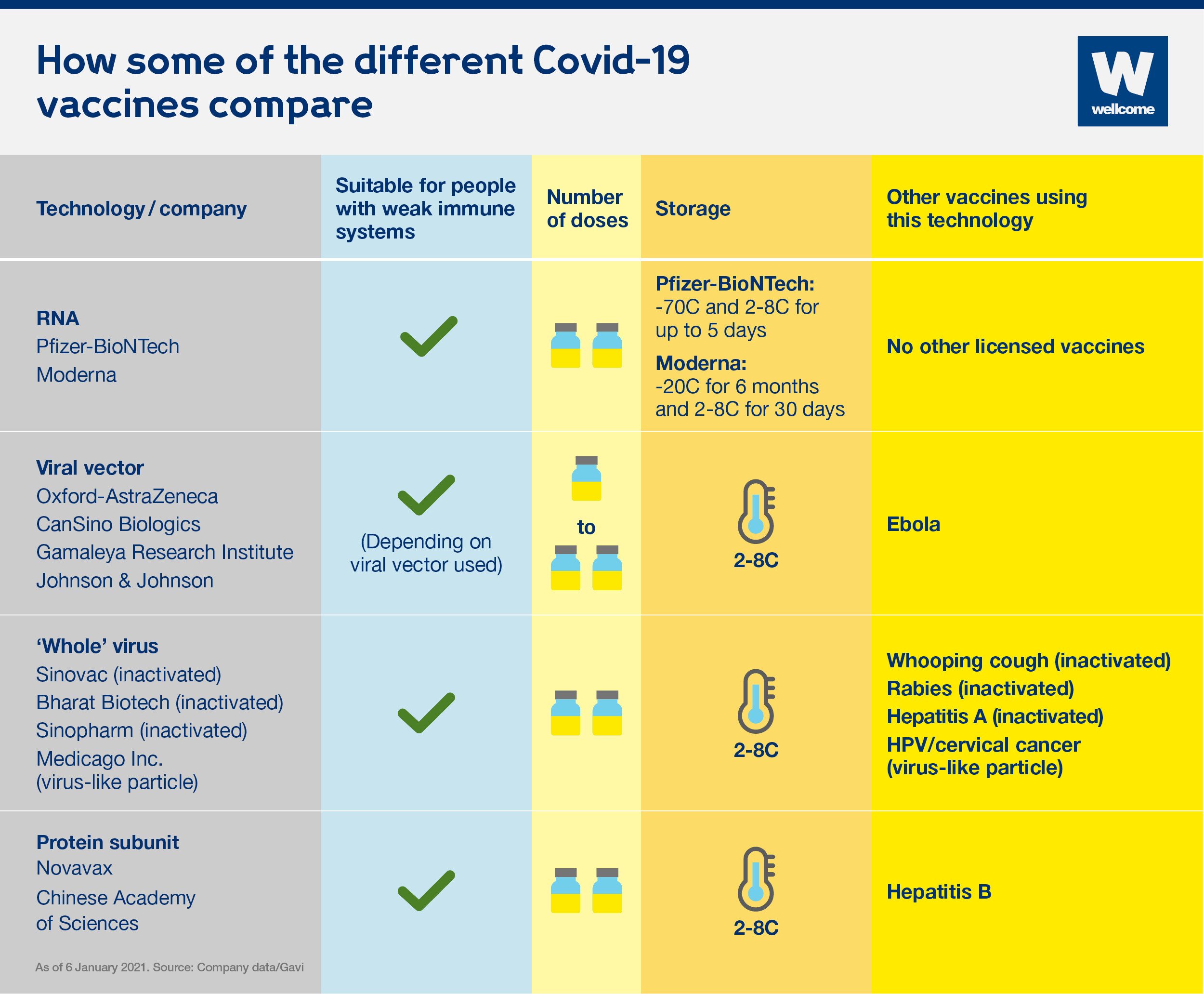
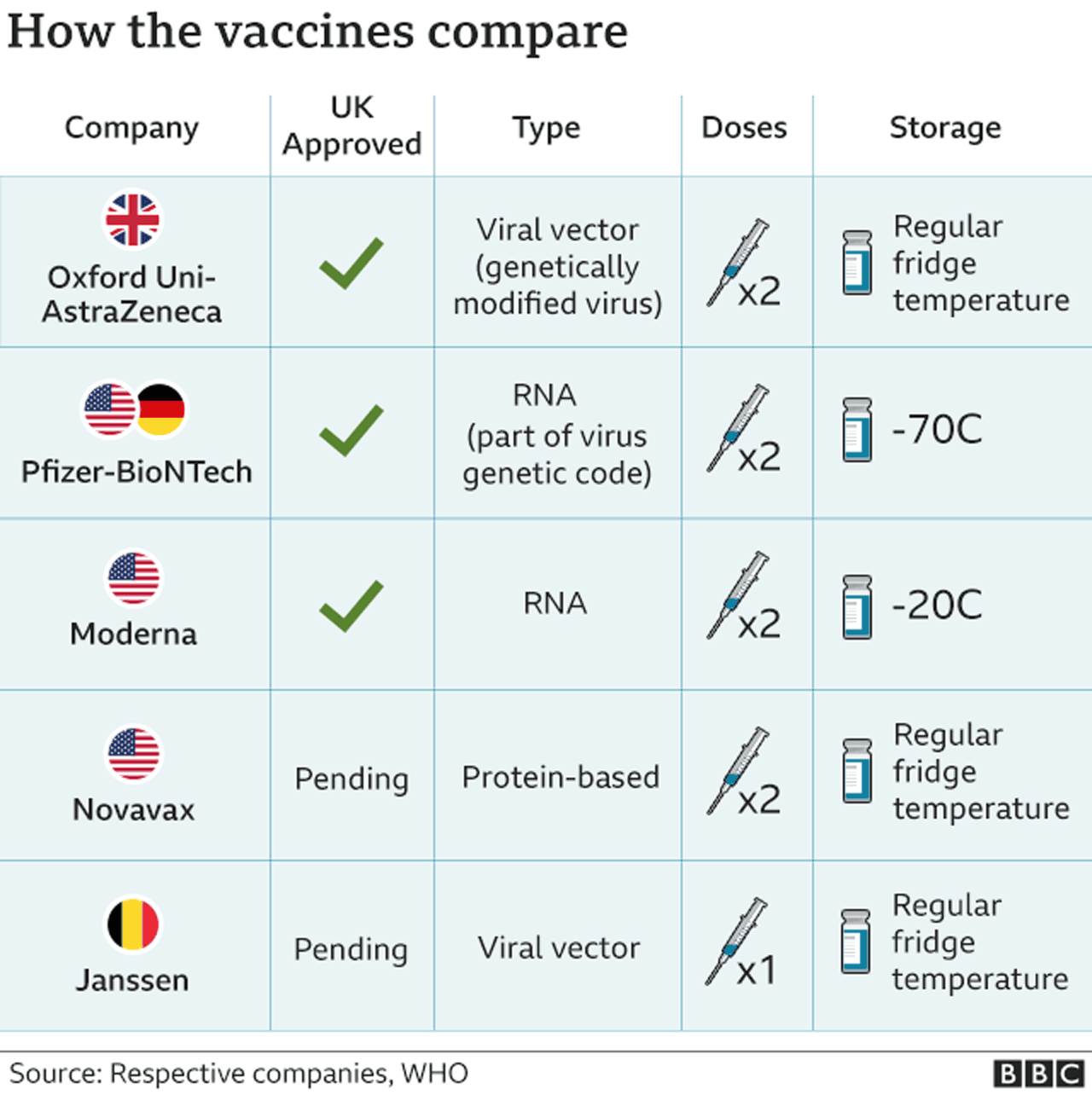
On September 23, the FDA authorized a booster shot of the Pfizer-BioNTech vaccine for people over the age of 65, individuals at high risk for severe disease and front-line healthcare workers six months after they received their second dose. The following day, the CDC added a group, recommending boosters for people between the ages of 18 and 64 who are at increased risk of COVID-19 due to their work or living situation. While all the COVID-19 vaccines authorized for use in the U.S. offer substantial protection, differences between them have been reported. In large clinical trials, the Pfizer-BioNTech and Moderna vaccines were each about 95% effective at preventing any type of COVID-19 illness, causing symptoms ranging from sniffles to death. In U.S. clinical trials, the Johnson & Johnson vaccine was reported as 85% effective at preventing severe disease and 72% effective at preventing moderate and severe disease. The vaccine was 66% effective overall in clinical trials held in the U.S., South African and Brazil.
COVID-19 vaccination data can be reported by countries as the total number of doses administered and/or the number of people vaccinated. Because most coronavirus vaccines require two doses, many countries also report the number of people who have received just one dose and the number who have been fully vaccinated. To provide greater insight into who is receiving the vaccine and racial/ethnic disparities in vaccination, KFF is collecting and analyzing state-reported data on COVID-19 vaccinations by race/ethnicity. These data will differ from survey estimates of vaccination rates that are limited to adults.
As far as the Delta variant, two studies reported by Public Health England that have not yet been peer reviewed showed that full vaccination after two doses is88% effectiveagainst symptomatic disease and 96% effectiveagainst hospitalization. But Israel later reported the vaccine's effectiveness to be 90% effective against severe disease, and 39% against infection in its population in late June and early July, based on an analysis of the country's national health statistics. Talk to your doctor about these concerns, but it's really important to get the third dose if you are immunocompromised. The latest research indicates the COVID-19 vaccines are safe and effective, but their effectiveness may wane over time and/or they may become less effective with new variants. Talk to your primary health care provider to determine the appropriate timing for your additional vaccination dose if you are immunocompromised or taking immunosuppressive therapies.
For one thing, the COVID-19 pandemic is now being driven by the delta variant, which is much more transmissible than previous variants. As of this week, federal data from the Centers for Disease Control and Prevention show that 78% of the adult population in the United States have received at least one dose of a COVID-19 vaccine. While this achievement has led to steep declines in COVID-19 cases and deaths, vaccination coverage—and the protections provided by it—remains uneven across the country. With the continued spread of the more transmissible Delta variant, unvaccinated people remain at increased risk for infection, illness, and death. However, the data show that these disparities are narrowing over time, particularly for Hispanic people.
On November 23, AstraZeneca and the University of Oxford announced high-level results from an interim analysis of their COVID-19 vaccine, AZD1222. The analysis was from the trials in the UK and Brazil and demonstrated efficacy of up to 90%. The vaccine was effective at preventing COVID-19, with no hospitalizations or severe cases in people receiving it.
There were a total of 131 COVID-19 positive cases in the interim analysis group. One dosing regimen was given at a half dose and demonstrated 90% efficacy, followed by a full dose at least one month apart. Another dosing regimen demonstrated 62% efficacy when two full doses were given at least one month apart.
The AstraZeneca vaccine can be stored, transported and handled at normal refrigerated conditions, about degrees F, for at least six months and administered within existing healthcare settings. People who are immunocompromised may continue to experience a reduced immune response to COVID-19 vaccines, even after receiving the third dose. It is important to continue following COVID-19 safety precautions, including wearing a mask in indoor public settings, staying apart from others who are not from your household, and avoiding crowds and poorly ventilated indoor spaces. Because the delta variant is highly contagious, social distancing and wearing high-quality KF-94 or KN-95 mask may offer better protection.
While the data provide useful insights, they also remain subject to gaps, limitations, and inconsistencies that limit the ability to get a complete picture of who is and who is not getting vaccinated. The completeness of race/ethnicity data has improved in most states over time as the shares of vaccinations with unknown or missing race have declined. However, some states still have relatively high shares of vaccinations among people classified with "unknown" race/ethnicity and three states still are not reporting vaccination data by race/ethnicity.
Moreover, although federal and state data are available for vaccinations by race/ethnicity and age separately, only a handful of states report data in a way that allows for analysis of vaccination rates by race/ethnicity within age groups. Having data to understand vaccination patterns by race/ethnicity and age will be particularly important when eligibility expands to children. Overall, comprehensive standardized data across states are vital to monitor and ensure equitable access to and uptake of the vaccine.
Vaccination rates are higher and gaps for Hispanic people close when analysis is limited to the population ages 12 and older who are currently eligible for the vaccines. Among those ages 12 and older, as of October 4, 2021, 64% of Hispanic people and 61% of White people had received at least one COVID-19 vaccine dose, compared to 55% of Black people. The narrowing of disparities in vaccination rates among Hispanic people when limiting to the eligible population reflects that a high share of children under age 12 who are not yet eligible for the vaccine are Hispanic. The gap in vaccination rates between Black and White people persists among the eligible population but is smaller than the gap among the total population.
More than 48.6 million additional doses have been administered worldwide, with many more countries expected to start administering them soon. The FDA based its approval on Pfizer's Biologics License Application , and its updated analysis of the safety and effectiveness of the vaccine in people aged 16 and older. The analysis studied 20,000 individuals who received the vaccine and 20,000 individuals who received a placebo and found that the vaccine is 91 percent effective in preventing COVID-19. The FDA found the most common side effects to be pain, redness and swelling at the injection site, fatigue, headache, muscle pain, chills, joint pain, and fever.
Soon, additional COVID-19 vaccine doses will be available for everyone in the U.S. to provide added protection against the coronavirus. Research studies show that immunocompromised people who have low or no protection following two doses of mRNA COVID-19 vaccines may have an improved response after an additional dose of the same vaccine. Efficacy is one of the key metrics reported in clinical trials that the FDA reviews when deciding to grant emergency use authorization. Efficacy metrics are also more reliable when a higher number of participants are involved in a clinical trial or study. The Pfizer and Moderna trials looked at prevention of any lab-confirmed infection starting at least seven to 14 days after the last vaccine dose in those who had symptoms.
However, the Janssen trial set a higher bar, looking to prevent moderate to severe or critical infection. "As we get more data about the kind of efficacy that they have over the longer term, we may see those numbers come down significantly for the mRNA vaccines," says Thompson. AstraZeneca-Oxford vaccine was 76% effective at preventing symptomatic COVID-19 two weeks after the second dose and was 100% effective in stopping severe disease and hospitalization in a U.S.-based clinical trial, according to the company.
Serious side effects, allergic reactions or adverse incidents stemming from the vaccines are rare, though in clinical trials, mild to moderate side effects were common. The most common complaints were pain at the injection site, fatigue and aching muscles and joints. Experts have found that many patients who had to be hospitalized after vaccination are immunocompromised. This updated emergency use authorization allows patients with organ transplants, and others who are immunocompromised, to get an extra dose of the same brand of shot they had received for their first two doses. Using a different brand of mRNA vaccine is allowed for the third shot if the brand previously received is not available.
The Moderna vaccine was 94.1% effective at preventing symptomatic Covid-19 after the second dose. The vaccine appeared to be equally effective across different ethnic and racial groups. The following map and chart show the number of COVID-19 vaccination doses administered per 100 people within a given population. Note that this is counted as a single dose, and may not equal the total number of people vaccinated, depending on the specific dose regime, as several available COVID vaccines require multiple doses. The State of California is currently drafting guidelines for distributing COVID-19 vaccines, beginning with highest risk groups, including health care workers and residents in skilled nursing facilities and to other essential workers.
The FCDPH will be partnering with health care providers to ensure the vaccine is available to everyone who is eligible to receive the vaccine, beginning with those at higher risk. If you want to receive the vaccine once it becomes available to the public, continue checking this webpage. The FCDPH has a separate website on the COVID-19 vaccine that is a source of information for health care providers. You can also consult with your medical provider to discuss when the COVID-19 vaccine might be available for you. The good news is that data from initial studies indicate that the three vaccines still work well against today's most common variants.
In the future, it's possible that new variants could emerge that lower a vaccine's effectiveness. New technologies that have made it easier and faster to create vaccines could help overcome this potential problem. Similar to an annual flu shot, we may need COVID-19 vaccine booster shots to protect against new virus variants. The Johnson & Johnson clinical trials took place months after the others.
By that time, virus variants were circulating in different parts of the globe. Some variants partially evade COVID-19 vaccines, perhaps accounting for the lower efficacy rates that have been reported. It's possible that, if all three COVID-19 vaccines were tested in the same way and at the same time, their efficacy rates would be similar.
The FDA has fully authorized Pfizer-BioNTech's COVID-19 vaccine, now called Comirnaty, for individuals age 16 and older. In addition, the Pfizer vaccine continues to be available under its emergency use authorization for individuals age 12 through 15 and for the administration of an additional third dose in immunocompromised individuals. Getting your booster shot once you're eligible will play a vital role in reducing your risk of COVID-19. The delta variant has shown us that we can't let our guards down and need to do everything we can to protect ourselves and each other – including immunocompromised individuals like our cancer patients at MD Anderson. The hope is that a booster shot will help amp up your body's immune response so it can better protect against COVID-19, even as new variants emerge. By comparison, a booster dose is a subsequent dose given months after the initial vaccination to reinvigorate the immune system.
Booster doses are intended for people whose immune response against COVID-19 may have decreased over time. In August, additional COVID-19 doses – also called third doses – became available for moderately and severely immunocompromised individuals. This is a third dose that comes after the initial two-dose vaccine series of the for Pfizer-BioNTech's or Moderna vaccine for people who may not have mounted a strong enough immune response after receiving the initial vaccine series.
Since these individuals have weakened immune systems, they need that third dose to protect against COVID-19 since they may not have had the same level of protection against COVID-19 as other people. Three different COVID-19 vaccines have been authorized for emergency use in the United States. All three have been proven to be safe and effective based on large-scale clinical trials. The answer to whether a booster shot is necessary or not should be based on the results of future phase III clinical trials. Preliminary results to date indicate that booster vaccination can successfully increase neutralizing antibody titer and antibody persistence.
It can also effectively improve the vaccine's ability to resist mutation. A new Chinese vaccine has recently been approved for public use in China. This vaccine is, like J&J's, a single-dose vaccine, and it's an adenovirus-based viral vector vaccine, similar to Sputnik V. CanSinoBIO's vaccine has shown 66% efficacy in preventing COVID symptoms and 91% in preventing severe disease. No serious blood clot cases had been reported in people inoculated with its single-dose COVID-19 vaccine. CanSinoBIO's vaccine is approved in China, Hungary, Chile, and Pakistan. Although Novavax entered the scene a bit late, it's one of the first vaccines to be thoroughly tested against newly emerging COVID variants.
Novavax is still not approved for public use, however, the company is expecting to receive emergency use authorization in the US and UK in May. Novavax is given in two spaced doses, just like the other variants mentioned earlier. Pfizer and BioNTech have announced that they will be holding clinical trials to see if a booster shot will be necessary with the growing concerns around the delta variant. Researchers around the world are creating and testing over 100 vaccines in an effort to combat the novel coronavirus. This chart breaks down the leading vaccines that have shown promise through pre-clinical data, completed Phase 1/2/3 clinical trials, and have been authorized for emergency use/ received approval. We also calculate the percent of the total population that has received a COVID-19 vaccine for 45 states that report racial/ethnic data based on people who have received at least one dose of the vaccine.
These data will differ from other estimates of vaccination rates among adults or the eligible population ages 12 and older. Today, the FDA issued the first emergency use authorization for a vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. A comment, though, regarding the AstraZeneca vaccine; it appears that the numbers you are using are for doses closer to the 12 week delay that is now recommended. The Lancet states that the efficacy for a delay of 6 weeks or less between doses is 55.1% but with 12 weeks or over, is 81.3%. Note that the efficacy of the AstraZeneca vaccine was never tested on the 56 + age cohort and the vaccine has not received approval in the U.S.
My vaccine appointment is tomorrow and I am seriously thinking of refusing it should it be AstraZeneca as I don't believe it should be offered to a cohort who was never studied. Isn't that why there are no plans to vaccinate children at this time, for lack of studies on that age cohort? The constant vaccinating positions of political and medical spokespersons, in this context, does not inspire confidence; surely this is a political decision. This dashboard displays various data on COVID-19 vaccines administered to Wisconsin residents. It provides breakdowns of vaccines administered by county, healthcare emergency readiness coalition region, age, sex, race, and ethnicity.
The dashboard also presents the total number of doses administered to Wisconsin residents each week. Data in this dashboard represent the county or HERC region in which the individual who was vaccinated lives, not where they received their vaccine. Total number of vaccine doses administered represents all COVID-19 vaccine doses that have been given by Wisconsin vaccinator or are a historical dose and reported to the Wisconsin Immunization Registry .
This includes doses administered through both the Federal Long-Term Care and Retail Pharmacy Programs, doses reported by Tribal health sites that receive vaccine from Indian Health Services , and some Veteran Affairs clinics. The total includes Wisconsin residents and non-residents who received a COVID-19 vaccine in the state. This tab also displays total administered doses according to vaccine manufacturer. India's Bharat Biotechannouncedin October 2021, that it had delivered one billion doses of its COVID-19 vaccine, Covaxin, in only nine months.