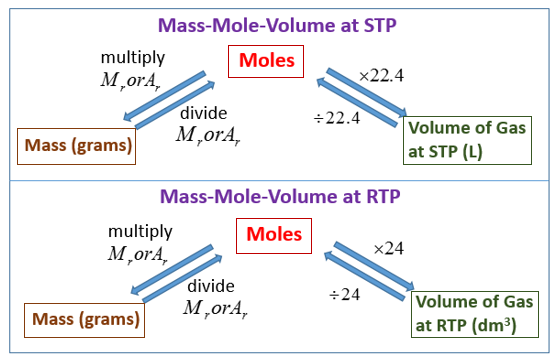
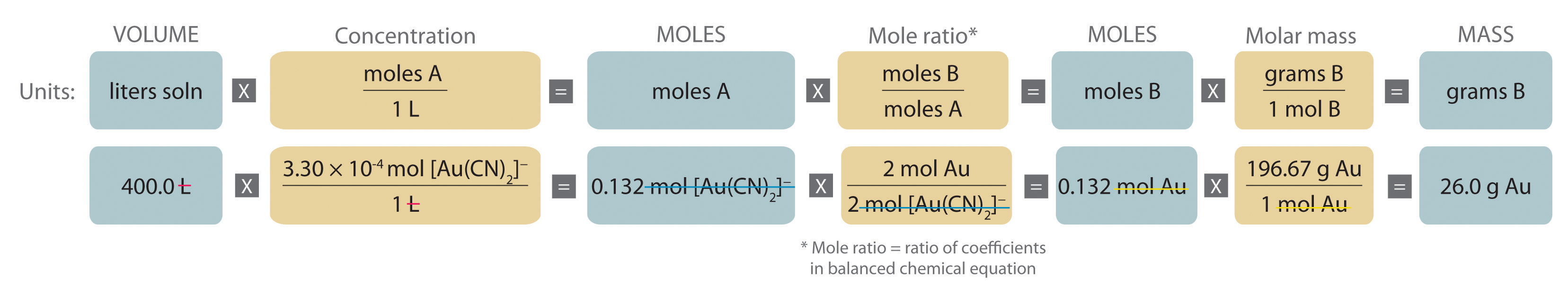
Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law. When products or reactants are gaseous in form at standard temperature and pressure, the molar volume of a gas and mole ratios can be used to convert the volume of one substance to moles of another substance. One mole of an ideal gas has a volume of 22.4 L at standard temperature and pressure.
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law).
Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law). For more accurate measurements, glassware that has been certified by standards agencies may be purchased. Table 3.2-2 lists the calculated volumes for one gram of water in air at atmospheric pressure at sea level for different temperatures, corrected for buoyancy with stainless steel weights of density 7.8 kg m−3.
The glass volumes are also calculated for the standard temperature of 20 °C, with small adjustments for borosilicate glass expansion or contraction with temperature changes. To have value, measurement results must be metrologically traceable to an appropriate reference, which in the cases treated in this chapter are SI units of mass, volume, and amount of substance. A statement of measurement uncertainty always accompanies a traceable result. Methods must be validated and verified for use by a particular operator at a particular time. Accreditation to an appropriate standard, such as ISO , is overseen by organisations usually with governmental or quasi-governmental status. Gaining accreditation for a particular method shows that a laboratory is using validated methods by competent personnel, but of course can never guarantee a reliable result .
In this section we will review the components of measurement uncertainty of mass and volume measurements and then apply this to the preparation of a standard solution and a typical titration. It is noted that the metrological traceability chain will involve multiple branches , often through amount fraction or mass fraction. For more information, see the chapter on quality assurance in the forthcoming 4th edition of the Orange Book , or in .
Avogadro was an Italian Physicist who first described the Avogadro constant as a hypothesis in 1811. He was trying to understand why in chemical reactions involving gases the observation that equal volumes of different gases had the same number of moles. This was found true even when the masses were very different.
The idea that a mole of any substance has exactly the same number of atoms no matter what the substance is made of was explained by Avogadro and his name has stuck to his number ever since. The interest stems from that accurate measurements of the unit cell volume, atomic weight and mass density of a pure crystalline solid provide a direct determination of the Avogadro constant. Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature.
In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures. For regular three-dimensional objects, you can easily calculate the volume by taking measurements of its dimensions and applying the appropriate volume equation. If it's an irregular shape, you can try to do the very thing that caused Archimedes to shout the famous word Eureka! Probably you heard that story - Archimedes was asked to find out if the Hiero's crown is made from pure gold or just gold-plated - but without bending or destroying it. The idea came to him when he was taking a bath - stepping into a bathtub, he noticed that the water level rose. From this observation, he deduced that volume of water displaced must be equal to the volume of the part of his body he had submerged.
Knowing the irregular object volume and its weight, he could calculate the density and compare it with the density of pure gold. Legend says that Archimedes was so excited about this discovery that he popped out of his bathtub and ran naked through the streets of Syracuse. Water displacement method was invented by Archimedes. This method is useful to calculate the volume of irregular solids, for eg, cone, cylinder, etc.
Volume is the quantification of the three-dimensional space a substance occupies. By convention, the volume of a container is typically its capacity, and how much fluid it is able to hold, rather than the amount of space that the actual container displaces. Volumes of many shapes can be calculated by using well-defined formulas. In some cases, more complicated shapes can be broken down into simpler aggregate shapes, and the sum of their volumes is used to determine total volume. The volumes of other even more complicated shapes can be calculated using integral calculus if a formula exists for the shape's boundary. Beyond this, shapes that cannot be described by known equations can be estimated using mathematical methods, such as the finite element method.
Alternatively, if the density of a substance is known, and is uniform, the volume can be calculated using its weight. This calculator computes volumes for some of the most common simple shapes. Measuring the volume depends on your object's state of matter. For liquids, you can use a graduated cylinder or burette for the chemistry lab measurements, or a measuring cup & spoon for everyday life purposes. For gases, to roughly measure the volume, you can inflate a balloon and use it to displace the water in a graduate cylinder. A similar method works for solids — put the object into a graduated container and measure the change in reading.
Is the volume occupied by one mole of a chemical element or a chemical compound. It can be calculated by dividing the molar mass by mass density (ρ). Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume.
The most common molar volume is the molar volume of an ideal gas at standard temperature and pressure (273 K and 1.00 atm). As you mixed the two liquids, the ethanol and water molecules began to interact with each other, packing together in a different way than in either pure ethanol or pure water. This results in a new density of the solution and thus, a volume that does not equal the sum of the two original volumes. In other words, the volume of solution, the denominator of our volume percent equation, does not equal the volume of solute plus the volume of solvent. In the case of water and ethanol, their mixture has less volume than the sum of the pure liquids.
End-point error – the systematic error occurring because the equivalence-point potential differs from the end-point potential under the given conditions of titration. The equivalence-point potential depends on the formal potentials of the analyte and titrant and on the number of electrons participating in half-reactions. When the transition potential, corresponding to the end-point, is close to the equivalence-point potential, the effect of the above-mentioned factors may be diminished. The measurement of mass is a central point of the quantification of material substances. A balance measures mass by sensing the weight force that presses an object down on the balance pan.
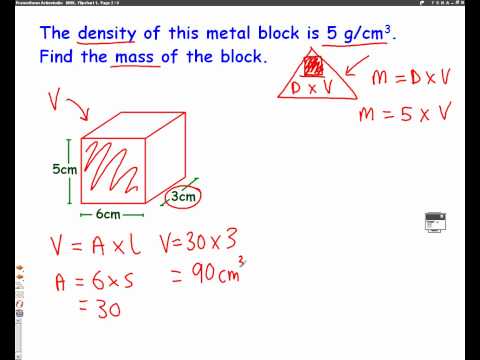
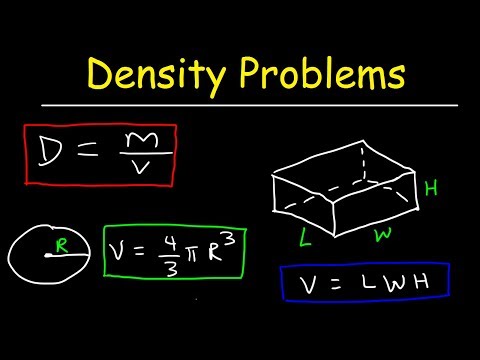
Weight is the force exerted on a body by the gravitational field of the earth, and is measured in the unit force newton, N. The weight force acting on 1 kg mass depends on geographic and cosmic factors. However, for mass measurements using mechanical balances, the weight of the unknown object is equilibrated at the same place and same time as the weight of an object of known mass (i.e. of a standard). For high precision measurements, the buoyancy caused by the surrounding air must be taken into consideration. This correction can easily be calculated if the density of the known and unknown mass and that of the air is known. The density of a substance can be used to define the substance.Water is unusual because when water freezes, its solid form is less dense than liquid water, and thus floats on top of liquid water.
The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. We can use the ideal gas equation to calculate the volume of 1 mole of an ideal gas at 0°C and 1 atmosphere pressure. Two solutions that have the same molarity will have the same number of molecules of the chemical per liter but are likely to contain differing masses of that chemical per liter to achieve this. Whereas two solutions at the same concentration will have the same mass of the chemical per liter of solution but are therefore likely to have differing numbers of molecules of that chemical per liter. Provided some additional information is known, one value can be deduced from the other using the equations below.
Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with 22.4 L at STP, and use molar mass and mole ratios to figure out how many products or reactants we have. Add the atomic masses of the solute together to find the molar mass. Look at the elements in the chemical formula for the solute you're using. List the atomic mass for each element in the solute since atomic and molar mass are the same.
Add together the atomic masses from your solute to find the total molar mass. In fact, gravimetric analysis was used to determine the atomic masses of many elements to six-figure accuracy. Standard solutions are often prepared by dissolving an accurately measured mass of a solute of certified purity in a known volume of solvent. If the concentration of an intended standard solution is obtained by measurement, for example by titration with a standard solution, it is known as a secondary standard [VIM 5.5]. Of a substance is the ratio of the mass of a sample of the substance to its volume.
The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, however, this as an inconvenient unit, and we often use grams per cubic centimeter (g/cm3) for the densities of solids and liquids, and grams per liter (g/L) for gases. Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 to 19 g/cm3 . Table \(\PageIndex\) shows the densities of some common substances. This formula is slightly tricky, with volume appearing multiple times.
Molar volume is a constant at room temperature and pressure . However, the volume of gas depends on what sample you are measuring. Just like how we can use molar mass to convert between the mass of a sample and its number of moles, we can use molar volume to convert between the volume of a gas and its number of moles. A small teaspoon of sodium hydrogencarbonate weighs 4.2g. Calculate the moles, mass and volume of carbon dioxide formed when it is thermally decomposed in the oven.
Assume room temperature for the purpose of the calculation. This means equal amounts of moles of gases occupy the same volume under the same conditions of temperature and pressure. This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 9.11. And even to estimate molecular weights, and there are many reports of such studies in the literature, as highlighted in the following section.
This means using the formula for the volume of a cylinder will produce an error in your calculation. Also, it's tricky trying to measure the internal diameter of the tube. The best way to find the volume of the test tube is to transfer the liquid to a clean graduated cylinder to take a reading.
Note there will be some error in this measurement, too. A small volume of liquid may be left behind in the test tube during transfer to the graduated cylinder. Almost certainly, some of the sample will remain in the graduated cylinder when you transfer it back to the test tube. The ideal gas equation contains five terms, the gas constant R and the variable properties P, V, n, and T. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm.
If the temperature is in kelvin, volume and temperature are directly proportional. Charles's law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant. Transition potential is often given instead of the formal redox potential. It corresponds to the colour change at which the end-point is said to occur. It is a function of the formal redox potential, the total concentration of the indicator , the depth of the colour layer, the minimal observable absorbance , and the absorption coefficient.
In an ideal two-colour indicator, the "apparent absorption coefficients" of both forms should be equal. Then the transition potential approaches the formal one. As for formal redox potential, it should be given, at least for the acidity range of indicator application. The transition potential may be given for pseudo-reversible indicators.
Because the transition point is usually different for oxidimetric and reductiometric titrations, it is sometimes useful to distinguish those two values. We know that density is equal to mass divided by volume and can be written as \[\dfrac\] . If we have the value of any two variables, the third variable can be calculated easily. But in this question only mass is given, so we will use the displacement method to find the volume. Take a measuring cylinder and add the given liquid to it and read the meniscus value which is the\[_\].
Now submerge the given object into the cylinder and let the object and water settle and read the meniscus again, this will give \[_\] . The \[_\] will be the final volume we need to find out. And, of course, you could redo this calculation to find the volume of 1 mole of an ideal gas at room temperature and pressure - or any other temperature and pressure. Given that carbon dioxide has a heavier molar mass than helium, packing 1 mol of carbon dioxide into the same volume means that the gas is denser. Indeed, the carbon dioxide balloon sinks while helium floats.
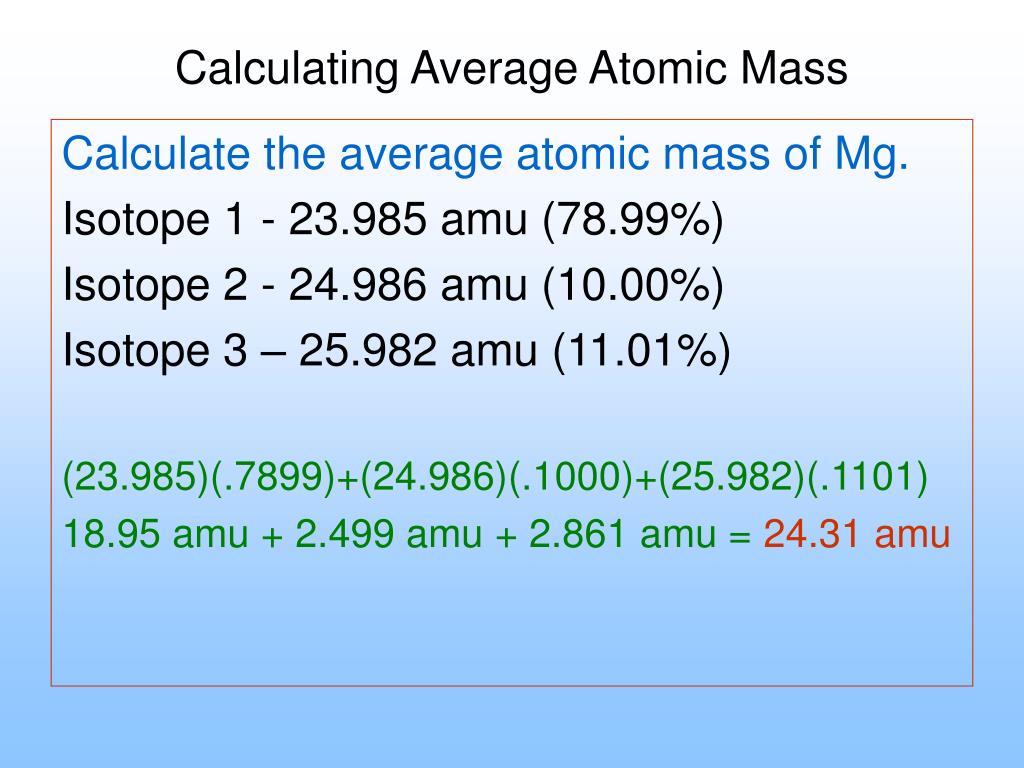
Whether you are counting hydrogen atoms, carbon dioxide molecules, or carbonate ions, a mole is always 6×1023. But as different particles have different mass, their molar mass varies. Values for atomic and therefore molecular masses are normally obtained relative to the mass of the isotope 12C (carbon-12), however "relative" is generally omitted from the title. Written correctly, the relative values have no units. The equation is read as 1 mole of calcium atoms reacts with 2 moles of hydrochloric acid to form 1 mole of calcium chloride salt and 1 mole of hydrogen gas molecules .
The relationship among the moles of a gas, the volume of the gas in liters, and the molar volume is similar to the mass-to-mass formula. In the sections above we calculated the w/v% concentration of solutions using the known mass of solute and volume of solution. (Add the atomic masses of the constituent elements.) Then, convert milligrams to grams by dividing by 1000. Finally, divide the grams of your substance by the Molar Mass. Because many objects are not regularly shaped their volume cannot be determined using a volume formula. The volume of these objects can be found by water displacement.
A volume of water sufficient to cover the object is placed in a graduated cylinder and the volume read. The object is added to the cylinder and the volume read again. The difference between the two volumes is the volume of the object. This method is demonstrated using the same battery used above. Acid and base aqueous reagents are usually described using weight percent (w/w%).




















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.